 Relevancy and Engagement
ny.agclassroom.org
Relevancy and Engagement
ny.agclassroom.org
In Search of Essential Nutrients (Grades 6-8)
Grade Level
Purpose
In this lesson students will learn the definition of an essential element, compare and contrast the essential nutrient requirements of plants and humans, explain why plants cannot use elemental nitrogen found in the atmosphere, and identify the sources for each essential nutrient needed by plants. Grades 6-8
Estimated Time
Materials Needed
Activity 1: Essential Nutrients
- 1 colored pencil per student
- Lesson handouts:
- Master 1.1, Essential Nutrients (Prepare an overhead transparency.)
- Master 1.2, The Periodic Table (Make 1 copy per student and prepare an overhead transparency.)
- Master 1.3, Chemical Symbols of the Elements (Make 1 copy per student.)
- Master 1.4, Essential Plant Nutrients (Prepare an overhead transparency.)
- Master 1.5, Essential Human Nutrients (Prepare an overhead transparency.)
Activity 2: Sources of Essential Nutrients
- Lesson Handouts
- Master 1.6, Sources of Essential Nutrients (Make 1 copy per student and prepare an overhead transparency.)
- Master 1.7, Using Nitrogen (Make 1 copy per student.)
Vocabulary
macronutrient: a nutrient that must be present in a relatively large amount to ensure the health of the organism; building blocks used to make essential biomolecules
micronutrient: a nutrient required in small quantities to ensure the health of the organism; often used as cofactors for enzymatic reactions
nitrogen fixation: a biological or chemical process by which elemental nitrogen, from the air, is converted to organic or available nitrogen
nutrient: a substance that provides nourishment essential for growth and the maintenance of life
nutrient deficiency: a condition where the amount of a nutrient essential to the health of an organism is lacking or present in an insufficient amount
Background Agricultural Connections
All organisms must take in matter from their environment in order to survive. There are 92 naturally occurring elements on Earth. Only a minority of them is needed by living things. For example, humans require about 21 different elements to be healthy. Almost all of the mass of our bodies comes from just six of those elements (carbon, hydrogen, oxygen, nitrogen, phosphorus, and calcium). These are the elements used to construct the carbohydrates, nucleic acids, proteins, and other molecules that make up our cells and carry out their chemistry. Other elements critical to our health are needed in very small amounts. Often, such elements are cofactors required by enzymes to catalyze specific chemical reactions. Regardless of whether elements are needed in large or small amounts, they must be obtained from the environment. Furthermore, it is not enough that essential elements are present in the environment; they must be available in a chemical form that our bodies can use.
Not surprisingly, the situation in plants is similar. They, too, must carry out thousands of different chemical reactions,many of which are similar to those of humans. Scientists have identified 17 elements that are described as essential elements (see Table 7). An element is described as being essential to the plant if the following conditions are met:
- The element must be required by the plant to complete its life cycle.
- The element can not be replaced by another element.
- The element must be required for a specific biological function.
- The element must be required by a substantial number of different plant species.
Three essential elements (carbon, hydrogen, and oxygen) are classified as non-mineral nutrients because they are obtained from the atmosphere and water. Three others (nitrogen, phosphorus, and potassium) are classified as macronutrients because they are needed in relatively large amounts. These six elements have important roles to play as building blocks for a cell’s biomolecules, such as proteins, nucleic acids, and carbohydrates. Three additional elements (calcium, sulfur, and magnesium) are called secondary elements, reflecting their supporting biochemical roles in the cell. The rest of the essential elements are called micronutrients because they are needed in small amounts. It is important to note that despite their name, micronutrients are just as essential to plants as are macronutrients.
The large majority of essential elements are absorbed by plants from water in the soil. Almost always the essential elements are taken up as a positively charged cation or a negatively charged anion. Of special interest is the situation regarding nitrogen. Although the atmosphere is about 80 percent nitrogen, plants cannot make use of nitrogen gas (N2 ). Instead, plants need to obtain their nitrogen by taking up the cation ammonium (NH4+ ) or the anion nitrate (NO3- ) in the soil. These ionic forms of nitrogen are generated by the breakdown of organic material in the soil or through a process called nitrogen fixation that is carried out by soil microbes. Some crop plants (legumes such as peas, beans, peanuts, and soybeans) live in close association with nitrogen-fixing bacteria that live in their roots and convert N2 gas to a form that plants can use. Such crops have a steady source of nitrogen and don’t require nitrogen-containing fertilizers.
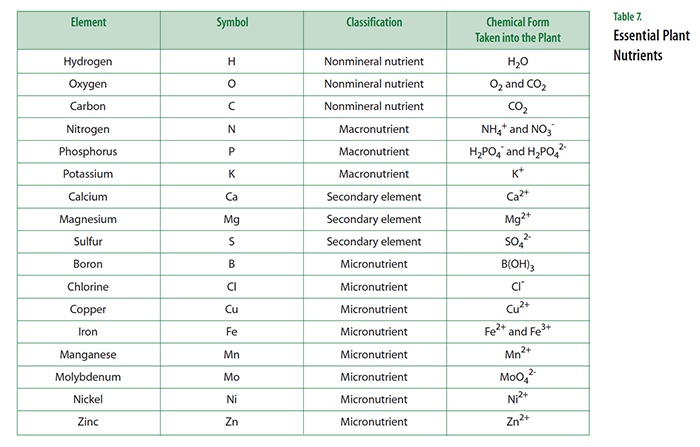
Teacher note: In this activity, the terms nutrient and chemical element are used interchangeably. In the context of plant requirements, carbon, oxygen, and hydrogen are called the non-mineral nutrients. Remember, it is not important to discuss each essential element; rather, you should focus on those elements that are important in building proteins, nucleic acids, lipids, and carbohydrates.
Engage
- Ask students questions to assess their prior knowledge. Questions could include:
- What is an essential element?
- What nutrients are essential to life? Are they the same for plants and humans?
- Where do these nutrients come from?
- After completing this lesson, students will be able to
- define an essential element,
- compare and contrast the essential nutrient requirements of plants and humans,
- explain why plants cannot use elemental nitrogen found in the atmosphere, and
- identify the sources for each essential nutrient needed by plants.
Explore and Explain
Activity 1: Essential Nutrients
- Begin the lesson by explaining that scientists who are interested in studying human health must understand the specific needs of the body. Ask students, “What do humans need to live?”
- Accept all answers. Write student responses on the board or on an overhead transparency. Direct the discussion to elicit air (oxygen), water, and food. Some students may realize that sleep is also required for survival. Other students may suggest environmental conditions such as temperature and pressure or material things such as clothing and shelter.
- Remind students that life requires energy for its existence. Ask students, “What do people take into their bodies from their environment to help them survive?”
- Students should recognize from their previous answers that air, water, and food are obtained from the environment.
- Ask students, “Why do we need air, water, and food to survive?
- Students should recognize that it is the oxygen in the air that we require.
- Students should be able to explain that our cells are mostly made of water. Water is the medium in which life has evolved. It is required for the chemistry of life.
- Students should recognize that we derive chemical energy from food and that it supplies the chemical building blocks needed by our cells.
- Remind students that humans (and animals) eat plants and other animals to obtain chemical energy and provide them with the building blocks needed by their cells. Ask students, “Do plants need food?”
- Keep in mind that ‘food’ is an imprecise term that includes both a source of chemical energy and nutrients. Some students may respond that plants do not need food because they can obtain energy from photosynthesis. Other students may mention that plants need water or that they obtain nutrients from the soil. If not mentioned by a student, remind the class that fertilizers can be considered food for plants.
- Explain that they will now investigate the chemical elements that are essential for plant growth. Display a transparency of Master 1.1, Essential Nutrients. Ask different students to read aloud the criteria that describe an essential element.
- There are more than 100 known elements that combine in a multitude of ways to produce compounds, which account for the living and nonliving substances we encounter.
- Pass out to each student a copy of Master 1.2, The Periodic Table and a copy of Master 1.3, Chemical Symbols of the Elements. Instruct the class to think about the definition of “essential element” and use a colored pencil to shade those elements on the periodic table that they think are essential for healthy plant growth. If possible, students should think of an example of how a given element is used by the plant (such as nitrogen being used to make protein).
- Give students about 5 minutes to complete this task. This step gives you an opportunity to assess how well students can relate their knowledge of chemistry to biology. For example, students may respond that carbon is used to make sugar. Students likely will not be able to suggest a function for elements needed in trace amounts. Usually, such elements are needed as cofactors for enzymes. It is not important to discuss the uses of each element, but it is important that students understand that these elements are needed to build cell structures and to carry out the cell’s chemistry through enzymatic reactions.
- Display a transparency of Master 1.2, The Periodic Table. Ask a student volunteer to read aloud the elements shaded on his or her periodic table. Have the volunteer explain why he or she selected those particular elements. Have additional students add to the list with their predictions.
- As the elements are read off, circle them on the transparency. Students are not expected to identify the complete list of essential elements. Their responses however, will reflect their relative knowledge about the biology of plants.
- Explain that you are now going to reveal which elements have been shown to be essential for plant growth and compare them with students’ predictions. Display a transparency of Master 1.4, Essential Plant Nutrients.
- Students likely will be surprised that so many elements are essential for plant growth. The comparison between the elements predicted by the students and the accepted ones should show some overlap, especially among the most abundant elements: carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorus (P), and sulfur (S). If not already mentioned, ask students to name an important molecule in the cell that requires the element phosphorus. If not mentioned, you can explain that the most important energy molecule in the cell is adenosine triphosphate (ATP) and it includes the element phosphorus. Cells carry on the many functions needed to sustain life. ...This requires that they take in nutrients, which they use to provide energy for the work that cells do and to make the materials that a cell or organism needs.
- Ask, “Do you think that humans require the same essential elements as plants?”
- Responses will vary. Some students may think that since humans and plants are very different from each other, they will need different sets of elements. Others may reason that since plants and humans are each made of cells, the essential elements needed by both will be similar.
- Display a transparency of Master 1.5, Essential Human Nutrients. Ask students to comment on how similar or dissimilar the pattern of elements is compared with that shown previously for plants.
- Students should notice that the two patterns are more alike than different. To make this point clearer, you can align and overlap the transparencies of Masters 1.4, Essential Plant Nutrients and 1.5, Essential Human Nutrients.
Activity 2: Sources of Essential Nutrients
Teacher note: This activity is designed to get students thinking about where plants obtain their essential nutrients. Some essential nutrients are obtained from more than one source. For the purpose of this activity, you want students to realize that plants obtain their non mineral nutrients (carbon, hydrogen, and oxygen) from the air and water, while the rest come from the soil.
- Explain that you will conclude the lesson with a brief activity that explores from where plants obtain their essential nutrients.
- Pass out to each student a copy of Master 1.6, Sources of Essential Nutrients. Explain that the handout lists the 17 essential plant nutrients. Instruct students to think about where a corn plant obtains its essential nutrients. Students should indicate the source—air, water, and soil—of each nutrient (that is, each chemical element) by checking the appropriate boxes on the handout.
- For the purpose of this activity, students should think about water as rainfall (before it reaches the ground). It therefore should not include those elements found in soil that may be dissolved in it. Students are free to check more than one box for any element. Give students about 5 minutes to complete this task.
- Display a transparency of Master 1.6, Sources of Essential Nutrients. Ask a student volunteer to describe which elements he or she listed as coming from water.
- Put a “W” next to the elements named by the students. Of course, students should mention hydrogen and oxygen. Actually, rainwater may contain small amounts of other elements derived from atmospheric gases and dust particles.
- Ask another volunteer to describe which elements he or she listed as coming from the air.
- Put an “A” next to the elements named by the students. Students should recognize that the corn plant obtains carbon (via CO2) and oxygen (via O2) from the air. Some students may know that most of the atmosphere is nitrogen (as N2). Most students will not realize that nitrogen gas is not available to the corn plant in a usable form. Do not correct this misconception yet. This issue will be addressed in Step 7. As with water, small amounts of other elements also may be present due to air pollution.
- Ask another volunteer to describe which elements he or she listed as coming from the soil.
- Put an “S” next to the elements named by the students. Students should list most if not all of the essential elements. The soil not only contains many elements that reflect its geological history, but it also contains organic material from once-living plants and animals as well as from the abundant microbial life that resides there.
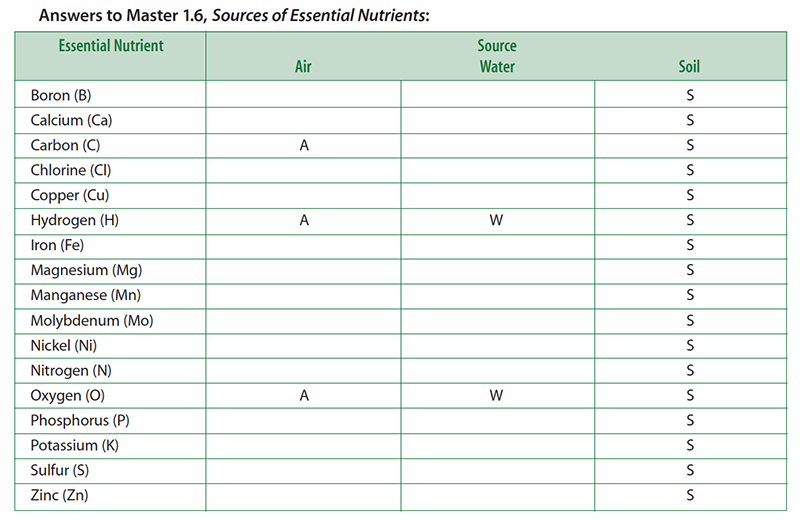
- Put an “S” next to the elements named by the students. Students should list most if not all of the essential elements. The soil not only contains many elements that reflect its geological history, but it also contains organic material from once-living plants and animals as well as from the abundant microbial life that resides there.
- Pass out to each student a copy of Master 1.7, Using Nitrogen. Instruct students to read the description and answer the questions.
- After students have completed their tasks, ask them, “In the light of what you just read, would you change your prediction of where the corn plant obtains its nitrogen?”
- Students should answer that the corn plant must obtain its nitrogen from the soil rather than from the air.
- Ask for a volunteer to read his or her answer to Question 1 on Master 1.7, Using Nitrogen.
- Question 1: What happens to plants if soil microbes are not present to either covert nitrogen gas to a usable form, or to release nitrogen from dead plants and the soil’s organic matter?
- Answer: Students should recognize that plants need nitrogen to survive. They should predict that the plants will get sick or die.
- Ask for a volunteer to read his or her answer to Question 2 on Master 1.7, Using Nitrogen.
- Question 2: What could you do to help crop plants grow in soil that doesn’t contain enough usable nitrogen?
- Answer: Students may suggest adding more microbes to the soil. Try to guide the discussion to the idea of adding nitrogen to the soil in the form of plant food (fertilizer), or occasionally planting legumes that have nitrogen-fixing microbes associated with their roots. If the question arises, be aware that non-crop plants may be adapted to very low nitrogen levels, in which case adding nitrogen would be detrimental.
- Ask students to help you summarize where the corn plant gets its essential elements. Likely student responses are the following:
- Water: Hydrogen and oxygen.
- Air: Carbon and oxygen.
- Soil: All essential elements.
- Conclude the lesson by summarizing that the plant obtains the nutrients carbon, hydrogen, and oxygen from the water and the air, while the rest are obtained from the soil.
- Explain that farmers need to know which essential elements are found in the soil and how much of each is present. Ask students to think of where the essential nutrients found in the soil come from.
- Student responses will vary. At this time, accept all answers. If not mentioned, use guided questions to bring out the fact that nutrient elements in the soil come from multiple sources that include:
- natural ones, such as the erosion of rocks;
- the action of lightning;
- the decomposition of plant and animal material, including soil organic matter (the dark layer at the soil surface);
- human-associated activities, such as organic and commercial fertilizer use by farmers and the public as well as from the waste that humans produce; and emissions from industry and automobiles
- Student responses will vary. At this time, accept all answers. If not mentioned, use guided questions to bring out the fact that nutrient elements in the soil come from multiple sources that include:
Elaborate
-
Dietitians use MyPlate to represent a healthy diet, balanced between the six food groups. Plants, too, must take in a balance of nutrients. Instruct students to prepare a “meal plan” for plants. Students should recall that plants obtain their essential nutrients from three sources: air, water, and soil. These three sources can be thought of as the plant’s food groups. Refer students to the sources for each essential element that they listed on Master 1.6, Sources of Essential Nutrients. The needed percentages from each food group (source) in their meal plan can be estimated by counting the number of elements from each food group and dividing by the total number of essential elements (17). For example, if a student listed just hydrogen and oxygen as coming from the air, then the percentage of needed nutrients from that group would be 2 ÷17 = 0.12 or 12 percent.
-
This lesson is the first in a series of five related lessons. Refer to the following lessons for further depth.
- Lesson 1: In Search of Essential Nutrients
- Lesson 2: Properties of Soils
- Lesson 3: Plant-Soil Interactions
- Lesson 4: Plant Nutrition Deficiencies
- Lesson 5: Fertilizers and the Environment
-
In Search of Essential Nutrients video clip.
Evaluate
After conducting these activities, review and summarize the following key concepts:
- Like humans and animals, plants also need a balance of nutrients to be healthy.
- Macronutrients are needed in relatively large amounts. Micronutrients are needed in relatively small amounts.
- Plants receive most of their nutrients from the soil.
- Many plants are produced by farmers which provide food for humans and animals. It is important to be knowledgeable of the nutrients plants need.
Sources
- Nutrients for Life Foundation
- BSCS-Biological Science Curriculum Study
- Reviewed by Smithsonian Institution