 Relevancy and Engagement
ny.agclassroom.org
Relevancy and Engagement
ny.agclassroom.org
FoodMASTER Middle: Eggs
Grade Level
Purpose
Students will learn the anatomy of eggs and the concept of forming colloidal dispersions called foams as they learn the anatomy of an egg, create a foam by whisking egg whites, investigate the effect of whisking time on foam, and compare and contrast the effect of different substances on the stability of foam. Grades 6-8
Estimated Time
Materials Needed
Student Materials
- Foaming Bonds student handout, 1 per student (Key)
- Foam Formulations student lab sheet, 1 per student (Key)
- Extraordinary Eggs student handout, 1 per student (Key)
Part A, per group of 4-5 students:
- 1 egg
- 1 egg separator
- 2 small glass or steel bowls
- 1 whisk
- 1 paper plate
- 1 kitchen timer or stopwatch
Part B, per group of 4-5 students:
- Assigned treatment. One of the following treatments pre measured and placed in a small cup
- 1 cup of egg whites
- 1 tsp. sugar
- ¼ tsp. salt
- ¼ Tbsp. acid (cream of tartar or lemon juice)
- ¼ tsp. vegetable oil (or any type)
- Note: Because there are 4 different treatments, multiple groups may be using the same treatment. Amounts above reflect what is needed for 1 group – adjust according. The plastic cups may be used to provide groups with their treatment.
- 1 funnel
- 1/8 cup (2 tablespoons) of egg white
- 1 small glass or steel bowl
- 1 rubber spatula
- 1 whisk or electric mixer
- 1 ruler
- 1 kitchen timer or stopwatch
- 1 10- or 25-mL graduated cylinder
- measuring spoons (teaspoon, ½ teaspoon, ¼ teaspoon, ½ tablespoon)
Vocabulary
colloidial dispersions: a substance in which particles are evenly dispersed within a medium (e.g., milk, because milk proteins and fat are dispersed in water)
foam: a frothy mass of tiny bubbles formed from a liquid, such as egg whites
solution: a type of homogeneous mixture in which the particles of one or more substances (the solute) are distributed uniformly throughout another substance (the solvent)
Did You Know?
- To tell if an egg is raw or hard-boiled, spin it! If the egg spins easily, it is hard-boiled but if it wobbles, it is raw.1
- The color of an eggshell is purely dependent on the breed of the hen.2
- Worldwide, around 1.2 trillion eggs are produced for eating every year. The average person consumes 173 eggs per year.2
Background Agricultural Connections
FoodMASTER (Food, Math and Science Teaching Enhancement Resource) is a compilation of programs aimed at using food as a tool to teach mathematics and science. For more information see the Background & Introduction to FoodMASTER. This lesson is one in a series of lessons designed for middle school.
| Weights & Measures | Fruits | Milk | Sugar | Protein |
| Food Safety | Vegetables | Cheese | Fats & Oils | Eggs |
| Energy Balance | Grains | Yogurt |
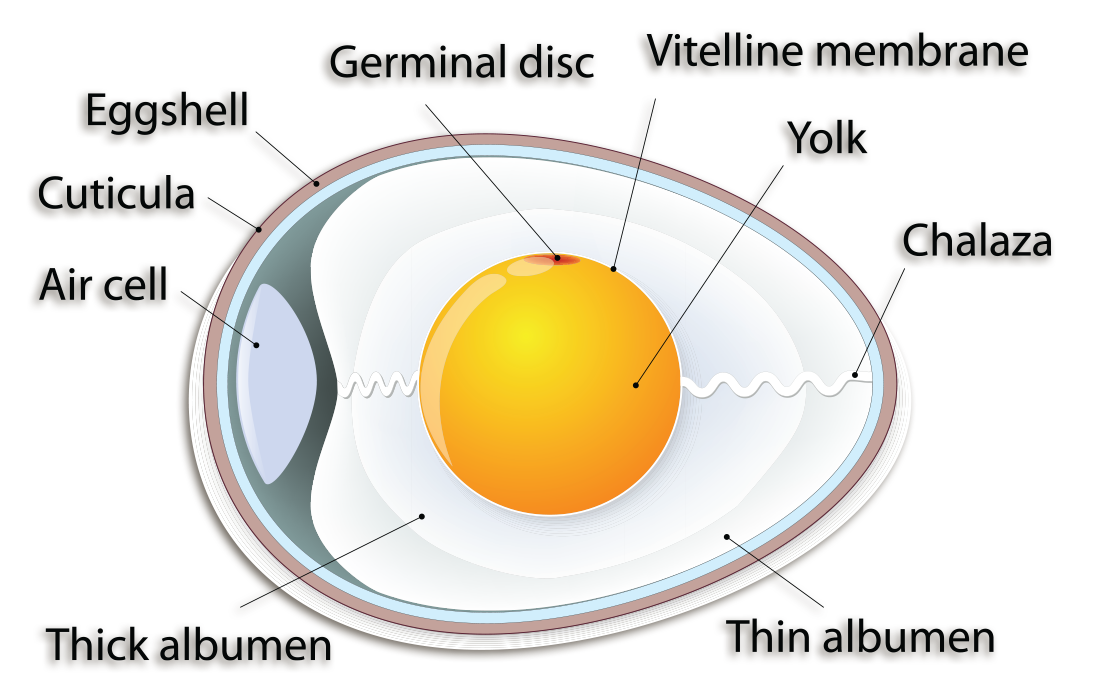 Eggs have an outer shell, a yellow yolk (vitelline), a clear white (albumen), and an air cell. The yolk contains most of the nutrients of the egg. The albumen contains mostly protein. On the side of the egg yolk, there is a small, white disk. This disk is referred to as the germinal disc. The twisted, cord-like structures on opposite sides of the yolk are called the chalazae [kuh-ley-zee]. Their function is to hold the yolk in the center of the egg white. An air cell forms after the egg is laid, as the contents cool and contract.
Eggs have an outer shell, a yellow yolk (vitelline), a clear white (albumen), and an air cell. The yolk contains most of the nutrients of the egg. The albumen contains mostly protein. On the side of the egg yolk, there is a small, white disk. This disk is referred to as the germinal disc. The twisted, cord-like structures on opposite sides of the yolk are called the chalazae [kuh-ley-zee]. Their function is to hold the yolk in the center of the egg white. An air cell forms after the egg is laid, as the contents cool and contract.
While chicken eggs are a source of protein in our diets, the egg’s purpose is actually reproduction. In a way, you can think of an egg like a single large cell, even though the yolk and white are molecularly multi-celled. The cell’s nucleus is located in the disc, and it is where cell division occurs in a fertilized egg. Embryonic development occurs here, making the germinal disc the ovum. The yolk and the albumen contain the nutrients for the developing embryo. The air cell is used to provide air to the chick from inside the egg. Keep in mind that the eggs we buy in the grocery store are not fertilized eggs and will never produce a chick.
Non-fertilized eggs are used for a variety of purposes in cooking. The egg’s emulsifying ability make it very useful in food preparation. The lipoproteins found in the yolk help form emulsions because some attract water and others repel water, which will allow oil and water to mix. The figure on the next page provides a visual of how oil and water do not mix naturally. The first picture (A) represents the separation of oil (dark gray) and water (light gray), while the last picture (D) represents a well-mixed solution. Liquids can also be mixed with gas to create a colloidal dispersion where the gas particles are evenly dispersed in the liquid.
When egg whites are beaten, a colloidal dispersion forms foam. The beating motion forces air (gas) into the egg white (liquid). When making foams, the addition of certain substances will change the characteristics of the egg foam. If sugar is added to egg white foam, it will increase beating time; however, a more stable foam will be produced due to a delay in the breakdown of protein. Acids, like cream of tartar, will decrease the pH and make the foam more stable. Salt will decrease stability, but enhance flavor. Fat will inhibit foam formation. Therefore, precautions should be taken to make sure your egg whites have not been contaminated with fat (e.g. oil or egg yolk). Acid and sugar are commonly used to make many egg-based recipes. For example, sugar is added when making meringues. 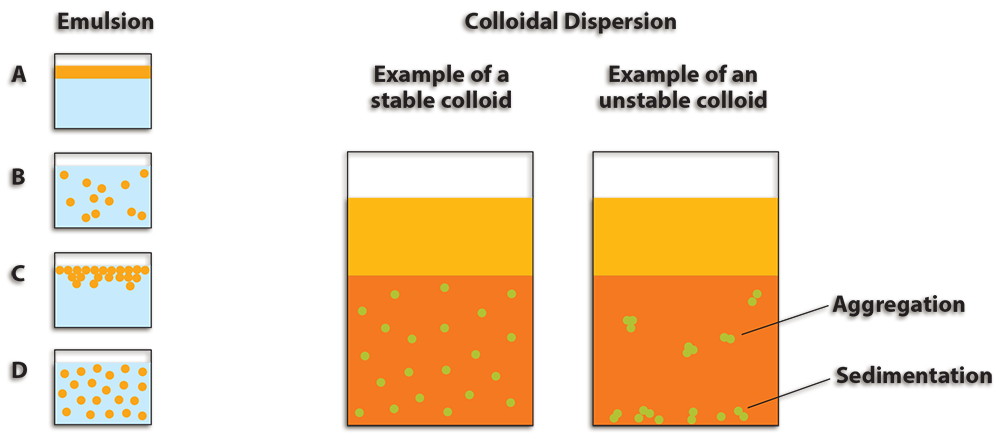
- Two immiscible liquids, not emulsified.
- An emulsion of "II" dispersed in "I."
- An unstable emulsion progressively separates.
- The surfactant positions itself on the interfaces between "II" and "I", emulsion stabilized.
Engage
- Show students a picture of lemon meringue pie. Ask, "What is the primary ingredient of the meringue in this pie?" Use guiding questions if necessary to lead students to recognize that meringue is made from egg whites.
- Ask, "Is it a chemical change or a physical change that transforms an egg white into the foam of a meringue?"
- Instruct students to define (or quickly research) the terms "foam" and "colloidal dispersion." Once the terms have been defined, ask how the terms can be related to eggs.

Explore and Explain
 Teacher Preparation
Teacher Preparation
- Review information found in the Background Agricultural Connections section of the lesson, lesson Procedures, and the attached Essential Files.
- Prepare materials for each group.
- For Part B, assign each student group a treatment (sugar, acid, fat, or salt).
- Prepare materials for treatment assignments. You are encouraged to allow students to measure out their own treatments; but if time is a concern, pre-measuring treatments is recommended.
- Egg White: 6 clear cups containing 1/8 cup of egg white
- Treatment A: 2 clear cups containing 1 teaspoon sugar
- Treatment B: 2 clear cups containing ¼ tablespoon (1/2 tsp. + 1/4 tsp.) acid (cream of tartar or lemon juice)
- Treatment C: 2 clear cups containing ¼ teaspoon fat (oil – any type)
- Treatment D: 2 clear cups containing ¼ teaspoon salt
- Separate the egg yolk and white ahead of time if you do not want students to participate in this portion of the lab. For Part B, you may also choose to use a carton of egg whites versus separating raw eggs.
- Frequent hand washing is an important emphasis in egg lab activities. Students should be discouraged from tasting raw egg (including foams). When practicing appropriate food safety procedures, eggs can be safely handled in the classroom.
- Timesaver: If feasible, students should use an electric mixer versus a whisk, particularly if time is a concern.
- Important Note: If you choose to separate the egg yolk and white to obtain the egg white needed for Part B, make sure the egg yolk does not contaminate the egg whites. Additionally, make sure no oils contaminate the bowl or beater. These factors can negatively impact the formation of egg whites during the investigation.
Lab: Foam Formulations
- Before beginning the lab investigation:
- Require students to wash their hands.
- For food safety reasons, DO NOT allow students to taste any egg products. When practicing appropriate food safety procedures, eggs can be safely handled in the classroom.
- Emphasize the importance of practicing good food safety behaviors by not consuming substances used as part of the lab investigation.
- Give each student one copy of Foaming Bonds and ask them to read the handout and complete the focus questions for this lab investigation.
- Distribute lab materials. It is recommended that materials are organized into stations for easier distribution. Students should be arranged in small groups of 4-5. Each group should receive the lab supplies outlined in the Materials section as well as 1 copy of the Foam Formulations lab sheet.
- Launch Part A of the lab by asking students to make a prediction about actions that can be taken to increase the stability (firmness) of egg white foams.
- Show students the Egg Cracking Demonstration Video. The video will demonstrate how to properly crack an egg and separate the white from the yolk.
- Next, show students the Foam Formulations Part A Video which will illustrate the four stages of peak formation in egg whites. The photographs found beginning on page 9 of the Student Demonstration Slideshow for illustration.
- Throughout Part A of this lab investigation, students will observe the parts of an egg and the different stages of foam formation in egg whites. This part of the lab will provide a foundation for Part B, when students observe how various substances impact foam formation.
- Allow students to work in small groups on the Foam Formulations lab sheet to complete Part A. After completing initial observations and conclusions, students should be prepared to begin Part B.
- Launch Part B of the lab by distributing the lab supplies outlined in the Materials section of the lesson. It is recommended that materials are organized into stations for easier distribution. Students should still be arranged in small groups of 4-5.
- Provide students with their assigned treatments. Students will predict what will happen to their egg white’s stability when their assigned treatment is added. Photographs demonstrating each treatment (before and after) are included in the Student Demonstration Slideshow.
- Sugar: When sugar is added to an egg white at the foamy stage it will create a smooth, stable foam. However, the addition of the sugar will delay denaturation of the egg proteins, increasing the beating time required to reach peak foam stage. Sugar is best added to egg whites in the beginning stages of foam formation.
- Acid: An egg white’s natural pH is basic (7.6 to 7.9). When an acid is added, it will decrease the pH of the egg white. Proteins are less stable and more prone to denaturation at lower pH levels. The denaturation of the egg proteins will improve foam formation; however, adding too much acid (pH less than 4.6) will result in delayed foam formation and decreased stability.
- Fat: Fat will cause a decrease in the stability of egg white foam. Even a small amount will interfere with formation of a foam. For this reason, the egg yolk cannot mix with egg white when making foams. The fat in the egg yolk will prevent the formation of foam
- Salt: Salt is often added to egg foams because it adds flavor. The addition of salt will decrease the stability of egg white foams if eggs are only beaten for a short time. If eggs are beaten for an extended amount of time, salt will not have an effect of the stability of the foam.
- Demonstrate how to measure the foam height or show students the Lab III: Foam Formulations Part B Video.
- Allow students to work in small groups to finish the Foam Formulations lab sheet.
- Follow-up with a class discussion about the impact of various substances on proteins.
Investigating Your Health Activity: Extraordinary Eggs
- Instruct students to research eggs and their health and/or nutritional benefits prior to beginning the activity.
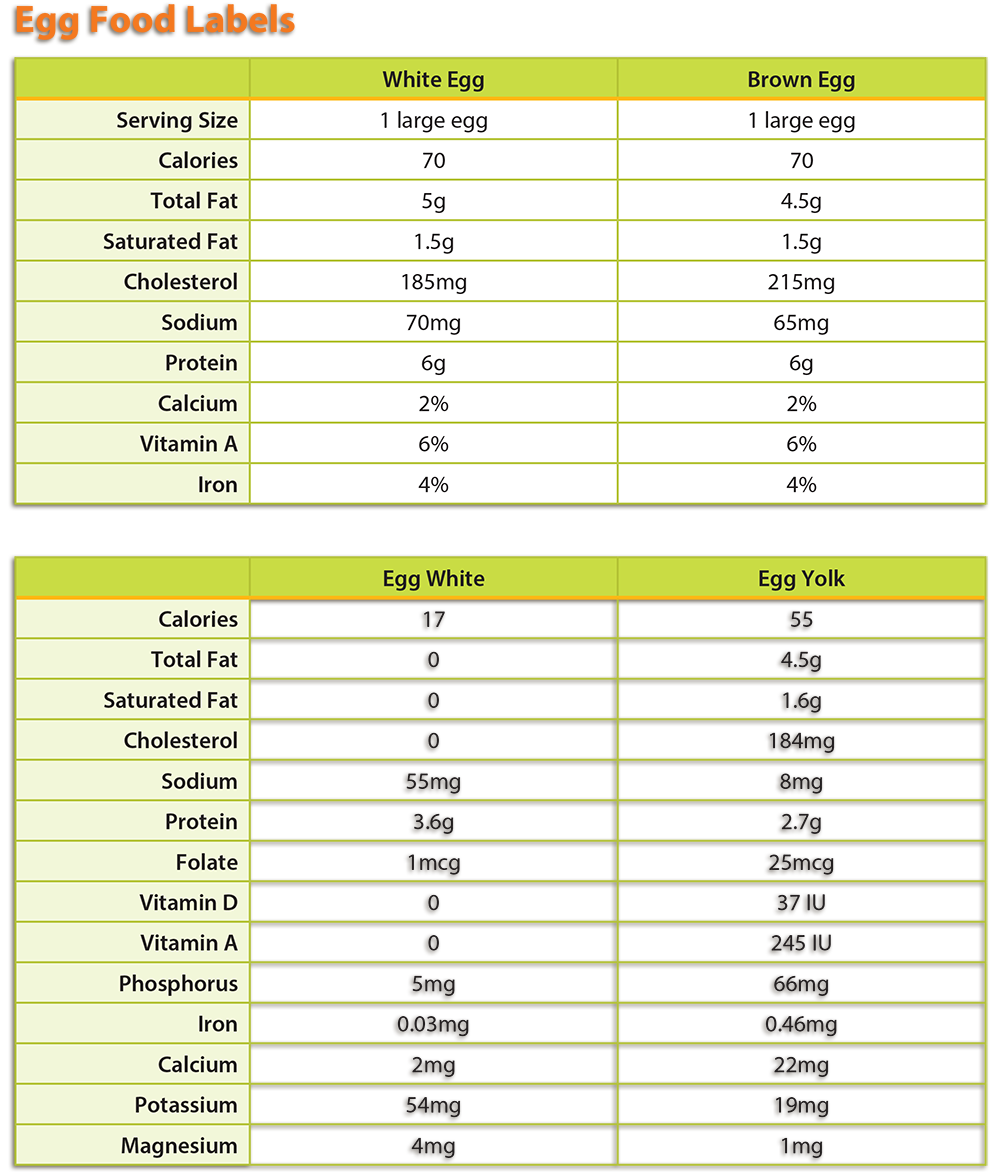
- Using the information provided in the Background Agricultural Connections section of the lesson or information learned from researching eggs, students should examine the nutrition profile for brown and white eggs and egg whites and egg yolks.
- Students can find the nutrition profile of eggs by accessing the USDA’s FoodData Central, or by using the label information in the graphic below.
- If you choose to use the provided egg nutrition information, see the attached teacher key for answers to the Investigating Your Health lab questions. Answers to questions should be similar despite the food label source used.
- If completed in-class, allow students to work in small groups on the handout to further explore the topic and respond to questions.
- Follow-up with a class discussion about student findings related to the health benefits of eggs.
Elaborate
-
Use egg white to make meringue cookies.
-
Use other types of foods to investigate emulsions (i.e. milk).
-
Investigate the impact of temperature (cold versus warm) on the formation of foams.
-
Investigate emulsifiers (see Activity 2: Examining Emulsions in the lesson FoodMASTER Middle: Fats and Oils).
Evaluate
After conducting these activities, review and summarize the following key concepts:
- Eggs provide a source of high quality and relatively inexpensive protein to our diet.
- Eggs are produced on farms, mostly by female chickens (hens) in the United States.
- Eggs can be consumed in many ways including such as scrambling, boiling, or frying. They may also be used as an important ingredient to many baked and processed foods.
Sources
- http://thinkegg.com/index.php/21-2/
- http://www.express.co.uk/life-style/top10facts/433957/Top-10-facts-about-eggs
Acknowledgements
This lesson was partnered with East Carolina University. The FoodMASTER program was supported by the Science Education Partnership Award (SEPA) which is funded from the National Center for Research Resources, a component of the National Institutes of Health.
- Primary Authors:
- Virginia Stage, PhD, RDN, LDN
- Mary White
- Ashley Roseno, MAEd, MS, RDN, LDN
- Melani W. Duffrin, PhD, RDN, LDN
- Graphic Design: Cara Cairns Design, LLC